Introduction: The JAK1/2 inhibitor, ruxolitinib, was approved in the USA in 2011 for the treatment of patients with myelofibrosis (MF) with intermediate and high-risk IPSS score (International Prognostic Scoring System). In the approval phase 3 COMFORT 1 - 2 studies, about 50% patients were taking ruxolitinib for at least 3 years, respectively.
Objective: We sought to evaluate the characteristics and outcome of MF patients on long-term ruxolitinib therapy (≥3 years) at our center.
Methods: We retrospectively reviewed the charts of patients with MF who were treated with ruxolitinib for ≥3 years. Cytogenetic were classified into risks according to Gangat et all, JCO, 2011. Descriptive statistics were used for nominal and continues variables, captured at the time of ruxolitinib start. Duration of therapy and overall survival (OS) were estimated using the Kaplan-Meier method, from the start of ruxolitinib initiation until the last day of initial ruxolitinib therapy, the date of last follow-up or death, respectively. Response to therapy was according to IWG-MRT 2013 criteria.
Results: Among 437 patients who initiated therapy with ruxolitinib at our center, 136 (31%) remained on therapy for ≥3 years and represent current cohort. Ninety-one patients (67%) were newly diagnosed; the remaining patients presented after a median of 28 months (range, 4-228) from MF diagnosis. Median time to initiate ruxolitinib from presentation to our center was 1 month (range, 0.3-123) for all patients. However, the time was longer for patients who presented > 3 months from MF diagnosis (median of 11.5 months; range, 3.5-123).
Patient's characteristics (n = 136) at the time of ruxolitinib initiation are summarized in Table 1. Median age was 67 years (range, 32-84), and 76 (56%) of patients were males. Half of the patients had high risk IPSS score, > 80% had systemic symptoms or splenomegaly. Eighty six percent of patients had diploid or favorable karyotype. JAK2 mutation was detected in 87% of tested patients.
Median duration of ruxolitinib therapy was 72 months (95% CI: 66-78). Over the median follow-up of 83 months (range, 36-174), 63 patients (46%) died. Currently, 48 (35%) patients are still on ruxolitinib; 88 discontinued therapy after a median time of 55 months (range, 47-63). By 5th and 7th year of therapy, out of 136 patients that were treated for at least 3 years, 35% and 65% percent of patients discontinued treatment. The reasons for discontinuation included allogeneic stem cell transplantation (SCT, n 5), cytopenia (n 6), progression of MF (n 38), progression to accelerate phase (n 2) or acute leukemia (n 7), patient's choice (n 11), and death (n 23: infection 4, cardiac 3, cancer 3, others 16).
Overall, 101 patients (74%) achieved IWG-MRT response, represented in majority by clinical improvement (CI) in spleen (n 90, 84%) and CI in TSS (n 51, 71%), respectively. The remaining patients achieved clinical benefit not qualifying for overall IWG-MRT response. Median duration of IWG-MRT response was 55 months (95% CI: 48-63). Responses were ongoing in 29 patients (29% of initial responders) at the time of last follow-up. Median duration of therapy was 75 months (95% CI: 68-82) for responders vs 60 months (95% CI: 39-79) for non-responders, p = 0.74.
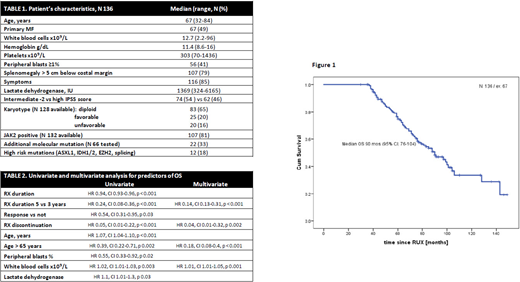
Median OS from the start of ruxolitinib was 90 months (95% CI: 76-104), Figure 1. Median OS for patients who were on ruxolitinib for ≥5 years (n = 73) was 106 months (95% CI: 80-137). Univariate and multivariate analysis for factors associated with OS is shown in Table 2.
After ruxolitinib discontinuation, 25 patients received subsequent treatment at our center: SCT in 6, another JAK inhibitor in 11, other investigational agents in 3, chemotherapy in 5 patients. Median OS from ruxolitinib discontinuation was 20 months (95% CI: 12-28).
Conclusion: Our data with the longest follow-up of patients receiving ruxolitinib for ≥3 years confirm the long-term benefit of this therapy with a median OS approaching 8 years since ruxolitinib treatment initiation.
Bose:Incyte Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Blueprint Medicines Corporation: Honoraria, Research Funding; Promedior, Inc.: Research Funding; Pfizer, Inc.: Research Funding; Kartos Therapeutics: Honoraria, Research Funding; Astellas Pharmaceuticals: Research Funding; Celgene Corporation: Honoraria, Research Funding; Constellation Pharmaceuticals: Research Funding; CTI BioPharma: Honoraria, Research Funding; NS Pharma: Research Funding. Pemmaraju:AbbVie: Honoraria, Research Funding; Incyte Corporation: Honoraria; MustangBio: Honoraria; Affymetrix: Other: Grant Support, Research Funding; Plexxikon: Research Funding; Celgene: Honoraria; Blueprint Medicines: Honoraria; Stemline Therapeutics: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Novartis: Honoraria, Research Funding; Pacylex Pharmaceuticals: Consultancy; LFB Biotechnologies: Honoraria; Roche Diagnostics: Honoraria; SagerStrong Foundation: Other: Grant Support; DAVA Oncology: Honoraria; Samus Therapeutics: Research Funding; Cellectis: Research Funding. Kantarjian:Daiichi-Sankyo: Research Funding; Ariad: Research Funding; Astex: Research Funding; Agios: Honoraria, Research Funding; Cyclacel: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; Takeda: Honoraria; Jazz Pharma: Research Funding; Immunogen: Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Research Funding; Pfizer: Honoraria, Research Funding; BMS: Research Funding. Verstovsek:Celgene: Consultancy, Research Funding; NS Pharma: Research Funding; AstraZeneca: Research Funding; Roche: Research Funding; Genentech: Research Funding; Novartis: Consultancy, Research Funding; Incyte Corporation: Consultancy, Research Funding; CTI Biopharma Corp: Research Funding; Promedior: Research Funding; Gilead: Research Funding; Blueprint Medicines Corp: Research Funding; PharmaEssentia: Research Funding; Sierra Oncology: Consultancy, Research Funding; Protagonist Therapeutics: Research Funding; ItalPharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal